X-Ray Medical Device Classification . — learn how medical devices are classified into four categories based on their risk and intended use, and what requirements and. outlines the process for classifying medical devices and explains how to seek clarification on classification of a medical. the manufacturer is responsible for applying the classification rules, which are determined by the products' intended. this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec. this document provides guidance for the application of the classification rules for medical devices as set out in annex ix of. this document provides guidance on how to classify medical devices based on their intended use and risk.
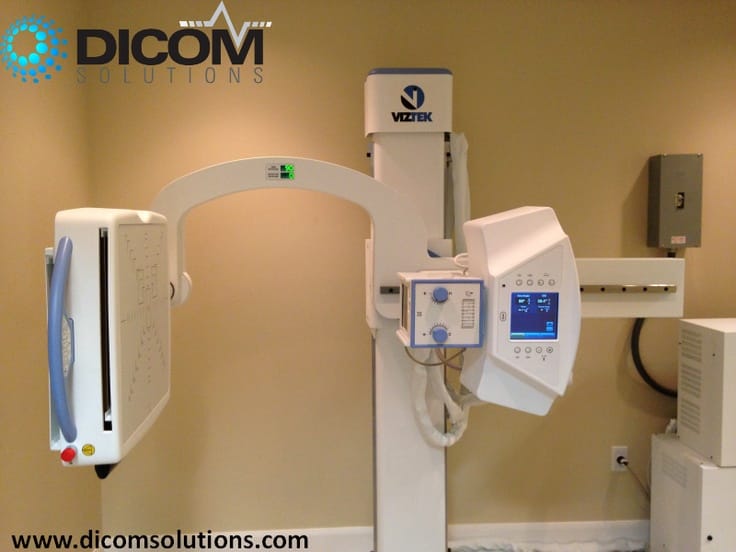
from dicomsolutions.com
this document provides guidance on how to classify medical devices based on their intended use and risk. the manufacturer is responsible for applying the classification rules, which are determined by the products' intended. this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec. outlines the process for classifying medical devices and explains how to seek clarification on classification of a medical. — learn how medical devices are classified into four categories based on their risk and intended use, and what requirements and. this document provides guidance for the application of the classification rules for medical devices as set out in annex ix of.
Different Types of XRay Equipment What is Best For You?
X-Ray Medical Device Classification this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec. — learn how medical devices are classified into four categories based on their risk and intended use, and what requirements and. the manufacturer is responsible for applying the classification rules, which are determined by the products' intended. this document provides guidance for the application of the classification rules for medical devices as set out in annex ix of. this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec. outlines the process for classifying medical devices and explains how to seek clarification on classification of a medical. this document provides guidance on how to classify medical devices based on their intended use and risk.
From middletownimaging.com
Digital Xray Middletown Medical Imaging X-Ray Medical Device Classification this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec. — learn how medical devices are classified into four categories based on their risk and intended use, and what requirements and. this document provides guidance for the application of the classification rules for medical devices as set out in. X-Ray Medical Device Classification.
From www.freepik.com
Premium Photo Xray medical professional equipment clinical X-Ray Medical Device Classification — learn how medical devices are classified into four categories based on their risk and intended use, and what requirements and. the manufacturer is responsible for applying the classification rules, which are determined by the products' intended. this document provides guidance on how to classify medical devices based on their intended use and risk. this document. X-Ray Medical Device Classification.
From www.intechopen.com
Xray Digital Tomosynthesis Imaging — Comparison of Reconstruction X-Ray Medical Device Classification outlines the process for classifying medical devices and explains how to seek clarification on classification of a medical. — learn how medical devices are classified into four categories based on their risk and intended use, and what requirements and. this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec.. X-Ray Medical Device Classification.
From pubs.rsna.org
Medical Devices of the Chest RadioGraphics X-Ray Medical Device Classification this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec. — learn how medical devices are classified into four categories based on their risk and intended use, and what requirements and. outlines the process for classifying medical devices and explains how to seek clarification on classification of a medical.. X-Ray Medical Device Classification.
From www.slideshare.net
Medical devices on the chest x ray X-Ray Medical Device Classification the manufacturer is responsible for applying the classification rules, which are determined by the products' intended. this document provides guidance for the application of the classification rules for medical devices as set out in annex ix of. outlines the process for classifying medical devices and explains how to seek clarification on classification of a medical. —. X-Ray Medical Device Classification.
From www.ce-marking.com
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark X-Ray Medical Device Classification — learn how medical devices are classified into four categories based on their risk and intended use, and what requirements and. outlines the process for classifying medical devices and explains how to seek clarification on classification of a medical. this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec.. X-Ray Medical Device Classification.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS X-Ray Medical Device Classification this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec. the manufacturer is responsible for applying the classification rules, which are determined by the products' intended. outlines the process for classifying medical devices and explains how to seek clarification on classification of a medical. — learn how medical. X-Ray Medical Device Classification.
From appadvice.com
Medical Devices on Chest XRay by RADIOLOGiQ, LLC X-Ray Medical Device Classification — learn how medical devices are classified into four categories based on their risk and intended use, and what requirements and. outlines the process for classifying medical devices and explains how to seek clarification on classification of a medical. this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec.. X-Ray Medical Device Classification.
From www.medrxiv.org
Chest Xray classification using Deep learning for automated COVID19 X-Ray Medical Device Classification this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec. this document provides guidance for the application of the classification rules for medical devices as set out in annex ix of. — learn how medical devices are classified into four categories based on their risk and intended use, and. X-Ray Medical Device Classification.
From www.freepik.com
Premium Photo Clinical radiography diagnostic device Xray medical X-Ray Medical Device Classification the manufacturer is responsible for applying the classification rules, which are determined by the products' intended. this document provides guidance for the application of the classification rules for medical devices as set out in annex ix of. this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec. —. X-Ray Medical Device Classification.
From www.gehealthcare.com
Xray GE Healthcare X-Ray Medical Device Classification this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec. this document provides guidance on how to classify medical devices based on their intended use and risk. — learn how medical devices are classified into four categories based on their risk and intended use, and what requirements and. . X-Ray Medical Device Classification.
From www.mi-3.co.uk
Your free guide to current MDR Classification Rules Mi3 X-Ray Medical Device Classification outlines the process for classifying medical devices and explains how to seek clarification on classification of a medical. this document provides guidance on how to classify medical devices based on their intended use and risk. this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec. — learn how. X-Ray Medical Device Classification.
From johnsonfrancis.org
Cardiac implantable electronic devices (CIED) on CXR All About X-Ray Medical Device Classification this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec. this document provides guidance for the application of the classification rules for medical devices as set out in annex ix of. — learn how medical devices are classified into four categories based on their risk and intended use, and. X-Ray Medical Device Classification.
From www.animalia-life.club
Endotracheal Tube Placement X Ray X-Ray Medical Device Classification outlines the process for classifying medical devices and explains how to seek clarification on classification of a medical. this document provides guidance for the application of the classification rules for medical devices as set out in annex ix of. this document provides guidance on how to classify medical devices based on their intended use and risk. . X-Ray Medical Device Classification.
From deepai.org
Deep learning classification of chest xray images DeepAI X-Ray Medical Device Classification this document provides guidance on how to classify medical devices based on their intended use and risk. this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec. outlines the process for classifying medical devices and explains how to seek clarification on classification of a medical. this document provides. X-Ray Medical Device Classification.
From media.creativeelectron.com
Why Xray Medical Devices Creative Electron, Inc. X-Ray Medical Device Classification the manufacturer is responsible for applying the classification rules, which are determined by the products' intended. outlines the process for classifying medical devices and explains how to seek clarification on classification of a medical. this document provides guidance on how to classify medical devices based on their intended use and risk. — learn how medical devices. X-Ray Medical Device Classification.
From www.behance.net
Medical device. Xray diagnostic system. on Behance X-Ray Medical Device Classification this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec. outlines the process for classifying medical devices and explains how to seek clarification on classification of a medical. this document provides guidance for the application of the classification rules for medical devices as set out in annex ix of.. X-Ray Medical Device Classification.
From www.medicaldevice-network.com
GE Healthcare launches fixed Xray system for radiologists X-Ray Medical Device Classification the manufacturer is responsible for applying the classification rules, which are determined by the products' intended. — learn how medical devices are classified into four categories based on their risk and intended use, and what requirements and. this document explains fda's policy and recommendations for industry on how to comply with eprc regulations and iec. this. X-Ray Medical Device Classification.